REDS Contributions to Science and Public Health
A summary of the REDS programs major contributions to science and public health follows:
1-Informing public health policies: Recent examples include performing the Transfusion-Transmitted Retrovirus and Hepatitis Virus Rates and Risk Factors Study, which demonstrated the feasibility of establishing a nationally coordinated, representative donor surveillance effort for known transfusion-transmissible infections (TTI) and their risk factors. The study was instrumental in helping develop the US TTI monitoring system (TTIMS) which collects data on HIV and hepatitis B and C for about 60% of the Nation’s blood supply; the program was established by the FDA in 2015. REDS-III also conducted the Blood Donation Rules Opinion Study which provided insight into non-compliance with the then current donor deferral policy (e.g., lifetime deferral) for men who have sex with men (MSM), the motivations of MSM who currently donate, attitudes and behaviors toward the donation screening process, and the likelihood of compliance with self-deferral under revised donor eligibility criteria. These studies were key in providing the necessary information to the HHS Secretary’s Advisory Committee for Blood and Tissue Safety and Availability (ACBTSA) and the FDA which resulted in a recommendation to change the MSM blood donor deferral policy in December 2014. In December 2015, the FDA Final Guidance for Industry was published with the deferral policy changed to allow MSM to donate after one year from last male-male sex. Other examples include establishing a multi-agency Blood XMRV Scientific Research Working Group (SRWG) and determining that XMRV was not a threat to blood safety (Simmons et al. Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study. Sciencexpress www.scienceexpress.org), and the current rapid response to the Zika virus epidemic. Four research studies are underway (or will start soon) domestically and in Brazil to evaluate Zika virus (as well as Dengue and chikungunya viruses) in blood donors and blood recipients that will inform worldwide blood screening policies and strategies for preventing transfusion transmission of these three viruses. Results from these studies are expected to allow transfusion medicine experts and government officials in Brazil and in the US to evaluate policy options for Zika, dengue, and chikungunya viruses, ensuring blood safety while being cognizant of costs associated with the implementation of new screening assays or pathogen inactivation procedures.
2-Establishing scientific approaches to assessing the risks of contracting transfusion-transmitted infectious agents: REDS has developed several statistical models to estimate the risks of transfusion-transmission by HIV and other infectious agents such as Dengue (Sabino et al. 2016 J Infect Dis. Mar 1;213(5):694-702). Most recently, REDS-III performed simulations to evaluate the robustness of HIV incidence models under various scenarios. These models are routinely used in the U.S. and internationally and have been since the late 1990s. Estimates derived from these models are used as part of current transfusion medicine practice to inform potential recipients of blood products of the risks of acquiring known transfusion-transmitted agents. These estimates are also an integral part of cost-effectiveness analyses conducted to evaluate current donation screening strategies. Finally, any evaluation of potential new strategies for reducing transfusion-transmitted risks in the U.S. or internationally today most often relies on the scientific approaches developed by the REDS program.
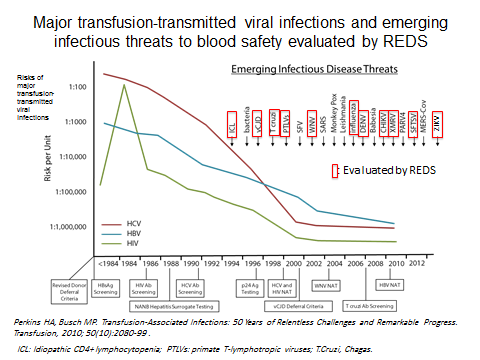
3-Mitigating transfusion-transmitted infectious risks through evaluation and implementation of improved donor screening methodologies: The risks of acquiring HIV or hepatitis C through transfusion have decreased from about 1:200,000-300,000 donations to 1 in 1.5-2 million donations over the past 15 years with much of the decline attributed to the implementation of nucleic amplification testing (NAT) based on data from studies conducted within the REDS program. REDS has conducted studies related to a number of other infectious agents, including hepatitis B, HHV-8, CMV, B19 virus, Human Pegivirus 2 (formerly GBV-c), influenza (seasonal and H5/N1), T. cruzi, and the arboviruses (West Nile virus, dengue virus, and now chikungunya and Zika viruses). For example, REDS played a major role in responding to the public health crisis that occurred in 2002 when clusters of transfusion- and transplant-associated WNV infections were first detected in the U.S. The rapid development and implementation of WNV RNA screening, within 8 months of the first reported transfusion cases and prior to the 2003 WNV transmission season, is a stellar example of government-academia-industry cooperation in a time of crisis. This process was greatly facilitated by the existence of the REDS collaborative network. The REDS group developed national protocols to monitor WNV NAT yield and to enroll and follow infected donors. REDS compiled the first year of WNV screening data from America’s Blood Centers (representing about 50% of the blood supply), established criteria for interpretation of screening and confirmatory test results, and contributed to studies that established the relative yield of minipool-NAT versus individual-donation NAT testing, and the rationale for targeted NAT testing of individual blood donors. Similar work has now been initiated for Zika virus.
4-Evaluating novel assays to detect the presence of transfusion-transmitted infectious agents: For example, REDS-III international programs have used and evaluated novel HIV-1 assays which can differentiate between recently and remotely acquired infections. These assays expand the ability to estimate incidence in repeat donors and permit the determination of incidence in first-time blood donors. This allows for calculation of residual risks, and thus, the ascertainment of blood safety in resource-poor countries where NAT is usually not available. Evaluation of these assays also inform HIV incidence work in general i.e., the results pertain to other populations. As a result of the REDS research on these assays, they have also been incorporated into the US TTIMS program.
5-Evaluating risk factors and characterizing the evolving epidemiology of infections in blood donors to inform broader population trends: Blood donor screening enables the identification of blood donors who are recently infected. This enables the evaluation of risk factors and characterization of the evolving epidemiology and genetic characteristics of viral infections. Understanding behavioral risk factors among recently infected donors allows not only implementation of appropriate deferral criteria and testing strategies to improve blood safety but also has broader public health implications for the country as a whole. Monitoring the circulating strains of viral infections has both direct blood safety benefit as well as broader public health benefit in all countries and regions where REDS programs are implemented. Additionally, early identification of HIV-infected donors in South Africa is permitting REDS-III to be uniquely situated to study the effect of antiretroviral implementation in the very early stages of HIV infection (ongoing REDS-III MATHS study).
6-Evaluating infectious disease burden in the general population: For example, REDS established the length of the West Nile Virus NAT-yield window period, based on NAT yield rates and seroincidence in North and South Dakota in 2003, and then used that information and national NAT yield data to project national WNV infection rates and to estimate the proportion of infections that develop serious neurological disease. A similar analysis has been performed by REDS-III for Dengue virus in Brazil and similar analyses are planned for Zika virus.
7-Assessing blood donor characteristics: Blood donors who are found to be infected with a transfusion-transmissible infection can be evaluated for their risk factors to inform how to improve donor screening strategies. Assessing donation patterns, rates of donor return, and blood availability determinants including what motivates and deters people from donating blood allows US blood centers to evaluate the effectiveness of ongoing donor recruitment and retention strategies and to determine where additional efforts are warranted to improve blood safety and availability.
8-Studies of iron metabolism: REDS has conducted several studies aimed at characterizing and evaluating how to improve iron status in blood donors. REDS-III conducted a randomized clinical trial (Kiss et al. 2015 JAMA 313(6):575-583) to determine the time to recovery of hemoglobin and iron stores in iron-replete and -depleted blood donors after whole blood donation (the study found that more than 56 days which is the allowed interdonation frequency is needed to recover) and evaluated what length of iron replacement therapy would be optimal to allow blood donors to recover post donation. REDS-III is also investigating the genetic characteristics of donor hemoglobin production and iron metabolism. One of the aims of the REDS-III RBC-Omics study which has enrolled over 13,000 blood donors is to evaluate the genetic characteristics of donor hemoglobin production and iron metabolism. Individuals who regularly donate blood as frequently as 10 times over a 24-month period without low hemoglobin deferral are a self-selected population with increased capacity for dietary iron absorption and maintenance of hemoglobin despite depletion of iron stores. These high-intensity donors represent a tremendous opportunity for studies of genes and proteins that regulate iron absorption and hemoglobin production. It is anticipated that results from these studies will help to further define how individualized donation intervals could be established and how adverse effects of iron deficiency in blood donors such as restless leg syndrome and pica can be avoided.
9-Reducing the risk of HIV transfusion-transmission and evaluating transfusion strategies in specific patient populations with HIV: The REDS program was the first to appreciate the value of “plasma donor seroconversion panels”, i.e., panels of plasma units derived from frequent serial donations from U.S. source plasma donors who were documented to have acquired HIV based on antibody seroconversion. Rigorous study of these panels yielded precise data on the evolution of viremia and humoral immune responses following acute HIV infection. These viral dynamics data were critical to the development of donor screening assays, most recently NAT, that were progressively implemented over the past 15 years in the U.S. and globally, resulting in reduction of residual risks of transfusion-transmitted HIV to 1 per 1.5-2 million. Equally importantly, these data were used to characterize early viral replication kinetics and establish a now widely employed system for staging acute HIV infection, ranging from the “eclipse”, “blip viremia” and “ramp-up” stages through progressive stages of seroconversion as documented by screening and confirmatory Western Blot assays and novel “recency assays” that are now widely employed to monitor HIV incidence worldwide. REDS studies of HIV seropositive donors in the domestic and international components have established the rate of detection and demographic correlates of HIV “elite controllers”, that is, seropositive individuals who have naturally suppressed viremia below the level of detection by ultrasensitive viral load and MP-NAT assays. REDS investigators confirmed that “elite controllers” are indeed infected based on extensive further testing for HIV plasma viremia and provirus, and established collaborations that are referring “elite controllers” as well as donors detected in the viremic pre-seroconversion window phase by NAT screening, to NIH funded programs for detailed studies of viral, host genetic and immunological mechanisms underlying pathogenesis. These studies have been critical to advancing the development of HIV vaccines and therapeutics. REDS-III international programs have implemented these HIV recency assays to determine recent versus remote infections and have conducted studies to determine temporal trends in HIV genotypes and drug resistance patterns. Additionally, REDS-III is conducting a study in South Africa to evaluate the effect of antiretroviral therapy in very early stages of HIV infection. Finally, REDS-III is evaluating the interactions between HIV infection and transfusion in patients with obstetrical hemorrhage in South Africa, as well as the reasons for low HIV incidence in patients with sickle cell disease in the US and Brazil.
10-Determinants of the erythrocyte response to storage, oxmotic and oxidative stress: The REDS-III RBC-Omics study is evaluating how ethnicity, gender, genetic, iron status, and metabolomic variation in blood donors may underlie the variable propensity of erythrocytes to hemolyze in vitro in response to routine RBC storage or in vitro stressors of hemolysis. Over 4,000 polymorphisms in hemoglobin, red cell membrane proteins and red cell enzymes known to affect RBC life span and stability have been identified, yet none have been comprehensively evaluated for their effects on red cell storage parameters. The results from this study will help evaluate how to improve RBC storage and inform our understanding of diseases with features of hemolysis such as sickle cell disease. As part of REDS-III, a transfusion medicine single nucleotide polymorphisms (SNP) and copy number polymorphisms (CNP) typing chip array was developed covering over 800,000 possible SNPs and CNPs related to blood and transfusion. The RBC-Omics samples will be investigated to determine if these genetic factors are associated with hemoglobin parameters, iron metabolism, red blood cell storage hemolysis propensity, and genetic and metabolomic profiles.
11-Epidemiology of blood component utilization in adults: REDS-III recently conducted a study to evaluate plasma utilization at 11 of the participating hospitals. This study which for the first time described plasma utilization in different adult patient services served as pilot for the development of the recipient database (one component of the vein to vein database) and informed the design of a clinical trial, currently being piloted through an R34. Similar analyses geared towards evaluating platelet and red blood cell utilization in adult patients are underway. REDS-III also established a collaboration with Kaiser Permanente Northern California to examine patterns of blood product utilization and predictors of RBC transfusion in hospitalized patients within an integrated health care delivery system. This retrospective cohort study utilized data on over 250,000 transfusions in 450,000 adult hospitalizations over a four-year period. These data represented about 20% of Northern California’s population who receive care at 21 hospitals and 56 outpatient clinics.
12-Evaluation of severe cardiopulmonary adverse events in adult transfused patients: REDS-III has evaluated the incidence of severe cardiopulmonary (with attention to transfusion-associated circulatory overload or TACO, and transfusion related acute lung injury or TRALI), septic, anaphylactic, acute hemolytic and hypotensive transfusion reactions in adult patients at 4 participating hospitals using active surveillance. Medical record review of 4,800 transfusions for cardiopulmonary reactions including TACO, TRALI, sepsis, anaphylaxis, and hypotension was conducted and results compared to the rate of routine operational reporting of these reactions. An automated electronic monitoring system was also developed and evaluated for its sensitivity and specificity in TACO diagnosis. Finally, a year-long case-control study of TACO risk factors is currently underway. Information on predictor variables (e.g., demographics, blood products transfused, infusion rates, fluid balance, diuretics and pressors, coexisting cardiac disease and other medical conditions) and outcomes (e.g., ICU and hospital stay and survival to discharge) are being collected and will be used to develop a predictive algorithm for TACO.
13-Evaluation of specific transfused patient populations
a. Establishment of a cohort of patients with sickle cell disease (SCD) in Brazil: In Brazil, SCD patients receive outpatient care in clinical facilities associated with blood banks (Hemocenters). REDS-III developed a comprehensive database of clinical, laboratory, and transfusion-exposure information from 2800 patients with sickle cell disease treated at the four participating REDS-III Brazil Hemocenters. This cohort will be followed for three years to: 1) define the incidence of specific SCD manifestations; 2) characterize blood utilization; identify rates and correlates of transfusion complications including alloimmunization, iron overload, and when it occurs, HIV infection; 3) determine the effect of chronic transfusion programs on clinical outcomes, such as reduced rates of stroke in the SCD population; 4) describe baseline laboratory parameters and their association with clinical outcomes; and 5) assess rates and causes of mortality. A biospecimen collection has been established and samples are being provided to TOPMed for full genome sequencing, as well as parallel SNP and CNP typing using the transfusion medicine array. The phenotype and genotype data will create an unmatched comprehensive dataset for the investigation of SCD outcomes.
b. Transfusion strategies in patients with obstetrical hemorrhage and HIV: In South Africa and other low/middle income countries, obstetric hemorrhage is a major cause of obstetric morbidity and mortality. A recently completed REDS-III study showed that although obstetric hemorrhage incidence in South Africa is not significantly increased compared to the US, the peripartum transfusion rate is ten-fold higher. A positive HIV status was associated with transfusion, even after controlling for age, parity and mode of delivery. Reasons for this may include antenatal anemia, coagulopathy and/or variability in institutional and physician practice. The completion of a pilot study has resulted in REDS-III performing a larger more detailed study to further examine this issue with enrollment of 1200 transfused obstetric patients as well as appropriate controls.
14-Establishment of resources for the scientific community:
a. Developing well characterized and sharable biospecimen repositories: REDS has developed large repositories of blood specimens to facilitate the conduct of research in transfusion medicine, hematology, infectious disease, and immunology. These repositories which are made available through BioLINCC can also be used to obtain well characterized biospecimens from a healthy population since blood donors are deferred if they have major health conditions. The most recent sharable biorepository that has been established in REDS-III consists of samples from over 13,000 blood donors who are currently being characterized for hemoglobin parameters, iron metabolism, red blood cell storage hemolysis propensity, and genetic and metabolomic profiles.
b. Establishing a vein to vein database prototype: The REDS-III domestic program has established for the first time a detailed research database infrastructure that links data from blood donors and their donations, the components made from these donations, and the recipients of these components; i.e., a particular donation can be traced through component production and, if transfused at a participating hospital, to a data extract from the electronic medical record of the transfusion recipient. Four years (2013-2016) of recipient data will be available once the database is finalized with an estimated 8 billion data points. For 2013, clinical and laboratory data (22 data tables with 319 variables) are currently available on 340,000 patient encounters (85% inpatients, 15% outpatients, 88% are ≥20 years old) of whom 17% received a transfusion (most commonly, red blood cells). Information on donors and their donations is currently available from mid 2012 to 2015. For 2013, preliminary linkage efforts resulted in >90% of transfused recipients data linked back to information on the corresponding blood donors and components. This database will permit the conduct of numerous analyses that characterize blood component utilization patterns in diverse settings, inform the design of future clinical trials, and determine potential blood donor/donation effects on adult recipients’ clinical outcomes. Examples of ongoing analyses include evaluating the effect of donor age and iron status on adult recipients’ clinical outcomes; investigating the effect of donor and component characteristics on the likelihood of recipient alloantibody formation; characterizing the epidemiology of platelet transfusion; identifying the donor and recipient risk factors associated with transfusion reactions; and determining whether red blood cell transfusion is associated with thrombotic risk.
c. Protocol-specific datasets: All major REDS study datasets are made available through BioLINCC to interested investigators.
15-Developing, diversifying, and sustaining a scientific workforce: REDS-III strives to foster the development of junior investigators who have an interest in epidemiology and laboratory research in transfusion medicine. A total of 39 junior investigators (18 in the domestic program, and 21 in the international programs) have been or are being mentored in the REDS-III program and have been empowered to lead several of the REDS-III projects or analyses under appropriate guidance. Many junior investigators have been paired with a mentor to together lead a REDS-III study or analysis thus providing practical hands-on experience as well as the ability to be lead author on resulting publications. Other mentoring activities have included: 1) one-on-one meetings with mentors; 2) mentoring on how to design and execute clinical and laboratory studies; 3) participation and leadership roles in REDS-III studies; 4) data analysis training supported by in-house statistical expertise and consultation with the DCC; 5) mentoring on preparation and writing of scientific manuscripts, abstracts, and posters, as well as resulting presentations; and 6) mentoring trainees in applying for extramural funded studies from industry, federal and philanthropic sources.
16-Generating additional hypotheses which have seeded NIH, CDC, and Bill and Melinda Gates Foundation grant-funded research: The HIV staging system, and further analyses using plasma donor seroconversion panels developed by REDS has fundamentally contributed to subsequent studies by R01-funded investigators and NIH-funded study groups [e.g., the Acute Infection and Early Disease Research Program (AIEDRP), the AIDS Clinical Trials Group (ACTG), the Center for HIV-AIDS Vaccine Immunology (CHAVI)] focused on very early HIV infection from pathogenesis, therapeutic, and vaccine development perspectives. REDS studies of hepatitis C (HCV) also informed HCV basic science and clinical investigators who now use HCV NAT to detect early HCV infections in a number of NIAID and NIDDK-funded studies focusing on the pathogenesis of early HCV infection. Additionally, spin-off R01 funding allowed studies of the rate, timing and mechanisms of HCV resolution. Additional research was generated following the establishment under REDS of a cohort of blood donors with Chagas disease in Brazil; this study established the incidence of Chagas cardiomyopathy in seropositive blood donors and investigated predictors of chronic infection and disease progression, including T cruzi parasite load and antibody assays, cardiac and inflammatory biomarkers, echocardiogram findings and host genetic markers. This research led to the current award by the Bill and Melinda Gates Foundation to support a treatment trial to evaluate biomarkers that reflect treatment efficacy, and an R01 from NIAID with the goal of improving and validating tests for spontaneous resolution and/or cure for T. cruzi infection. Other examples of spin-off funding in non-infectious areas include one R21 validating the link between NXPH2 (neurexophilin 2) and alloimmunization that emerged from a REDS-III study evaluating the genetic determinants of alloimmunization using biospecimens collected in REDS-II (and housed by BioLINCC), an R01 funded clinical trial to evaluate different strategies to reduce iron deficiency in blood donors (STRIDE), and most recently, a new R01 to conduct additional epidemiological analyses using the Northern California Kaiser databases.
